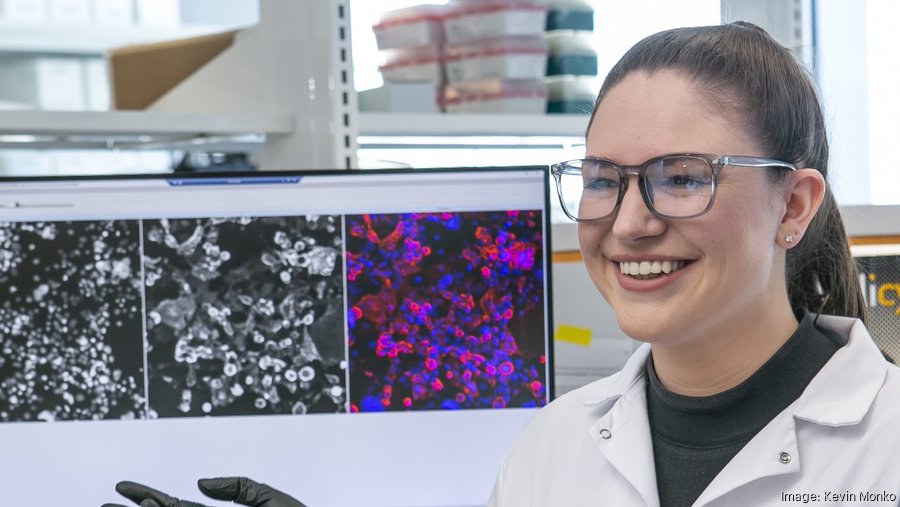
The Wistar Institute is investing $24 million on a campus expansion project that will create an HIV Cure and Viral Disease Center. The center will be housed on a new Wistar North campus at 3675 Market St. in University City and feature more than 25,000 square feet of laboratory and office space.
New Report Documents Momentum in Greater Philadelphia’s Cell and Gene Therapy Ecosystem
Nearly 10 percent of the world’s cell and gene therapy companies are based in Greater Philadelphia, and the industry now employs more than 7,000 people in the region, a sign of the depth and breadth of this fast-growing industry, according to a study commissioned by the Chamber of Commerce for Greater Philadelphia.
Integral Molecular spinout targets $10 billion antibody reagent market
Cell Surface Bio was spun out of Integral Molecular, a 23-year-old University City biotechnology company that uses its expertise in targeted biology antibody discovery, virology, and protein engineering to help drug developers advance potential new therapies for difficult to treat diseases.
Pioneering The First Cell Therapy For Solid Tumors
Back in February, the FDA approved the first cell therapy for solid tumors — Iovance Biotherapeutics’ Amtagvi (lifileucel). Amtagvi is an autologous T-cell therapy — or more specifically, a tumor-infiltrating lymphocyte (TIL) therapy (the first TIL therapy to be approved by the FDA) — for patients with previously treated, unresectable or metastatic melanoma. Learn what sets TILs apart from its other T-cell therapy counterparts and about Iovance’s preparation leading up to this monumental approval.